Crohn’s Disease and the Gut Microbial Changes You Can’t See
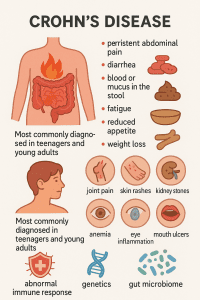 Crohn’s disease is a chronic systemic condition that causes inflammation to develop anywhere along the digestive tract. It is a specific type of inflammatory bowel disease, or IBD, and most often affects the small intestine and colon. It can develop at any age but is most commonly diagnosed in teenagers and young adults.
Crohn’s disease is a chronic systemic condition that causes inflammation to develop anywhere along the digestive tract. It is a specific type of inflammatory bowel disease, or IBD, and most often affects the small intestine and colon. It can develop at any age but is most commonly diagnosed in teenagers and young adults.
The ongoing inflammation associated with the condition can lead to symptoms including persistent abdominal pain, diarrhea, blood or mucus in the stool, fatigue, reduced appetite and weight loss. Over time, this inflammation can damage the gut lining, disrupting digestion and making it harder for the body to absorb essential nutrients. Symptoms can range in severity and often come and go in periods of flare-ups and remissions. While symptoms generally develop gradually, they can sometimes appear suddenly, without warning.
In addition to digestive issues, individuals with severe Crohn’s disease may also experience symptoms outside the gut, such as joint pain, skin rashes, kidney stones, anemia, eye inflammation and mouth ulcers. Notably, in children, Crohn’s disease may even delay physical growth and sexual development.
While the exact cause isn’t yet fully understood, Crohn’s disease is thought to result from an abnormal immune response, genetics, and environmental factors all working together. More recently, scientists have also been exploring the role of the gut microbiome in the pathogenesis of the condition. Disruptions in this delicate microbial balance may help to drive inflammation, making the microbiome a key focus in Crohn’s research and potential treatment.
How Crohn’s and the Gut Microbiome Interact — and Why It Matters
Crohn’s disease and the gut microbiome can influence each other in significant and often unpredictable ways. The inflammation that Crohn’s disease causes can throw off the balance of microbes in your gut, while shifts in these microbial communities might actually trigger or worsen the inflammation. This imbalance, known as dysbiosis, is a key feature seen in many people with Crohn’s.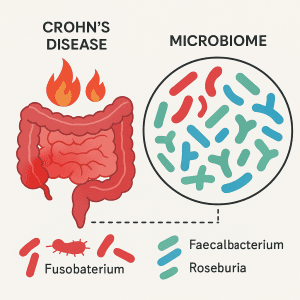 An altered gut microbial composition has been consistently observed in patients with Crohn’s disease and is believed to contribute to the initiation and perpetuation of intestinal inflammation. One of the most consistent features is a reduction in microbial diversity, meaning there are fewer types of bacteria living in the gut (1) In a study describing the distinct microbial signature of Crohn’s disease, researchers reported lower levels of beneficial bacteria such as Faecalibacterium, Anaerostipes, Methanobrevibacter, and unknown members of the Peptostreptococcaceae and Christensenellaceae families, alongside higher levels of potentially harmful bacteria including Collinsella, Fusobacterium, and Escherichia.(1) Studies also show that Klebsiella pneumoniae is more prevalent in the gut microbiota of individuals with Crohn’s disease compared to healthy controls.2() K. pneumoniae is a harmful bacterium that can colonize the oral cavity and subsequently enter the gut, fueling inflammation. In genetically susceptible people, Klebsiella is believed to trigger strong immune responses, including increased antibody production and Th1-driven inflammation, which may further drive Crohn’s disease activity. Changes in the gut microbiome can be detected even in the early stages of Crohn’s disease, with reductions in beneficial microbes including Roseburia, Gemmiger, and Coprococcus, Ruminococcus (2), Butyricicoccus, Dorea, Fusicatenibacter, Anaerostipes, Clostridium cluster IV, and an increase in others such as Parabacteroides and Lachnospiracea incertae sedis.(3) These shifts suggest that microbial imbalance is not just a consequence of long-term inflammation but may play a role from the very beginning.
An altered gut microbial composition has been consistently observed in patients with Crohn’s disease and is believed to contribute to the initiation and perpetuation of intestinal inflammation. One of the most consistent features is a reduction in microbial diversity, meaning there are fewer types of bacteria living in the gut (1) In a study describing the distinct microbial signature of Crohn’s disease, researchers reported lower levels of beneficial bacteria such as Faecalibacterium, Anaerostipes, Methanobrevibacter, and unknown members of the Peptostreptococcaceae and Christensenellaceae families, alongside higher levels of potentially harmful bacteria including Collinsella, Fusobacterium, and Escherichia.(1) Studies also show that Klebsiella pneumoniae is more prevalent in the gut microbiota of individuals with Crohn’s disease compared to healthy controls.2() K. pneumoniae is a harmful bacterium that can colonize the oral cavity and subsequently enter the gut, fueling inflammation. In genetically susceptible people, Klebsiella is believed to trigger strong immune responses, including increased antibody production and Th1-driven inflammation, which may further drive Crohn’s disease activity. Changes in the gut microbiome can be detected even in the early stages of Crohn’s disease, with reductions in beneficial microbes including Roseburia, Gemmiger, and Coprococcus, Ruminococcus (2), Butyricicoccus, Dorea, Fusicatenibacter, Anaerostipes, Clostridium cluster IV, and an increase in others such as Parabacteroides and Lachnospiracea incertae sedis.(3) These shifts suggest that microbial imbalance is not just a consequence of long-term inflammation but may play a role from the very beginning.
Similarly, a microbiome study involving children newly diagnosed with Crohn’s has helped to pinpoint consistent shifts in gut bacteria before treatment begins. This large pediatric study found a distinct microbial signature associated with the disease, which included an increase in Enterobacteriaceae, Pasteurellaceae, Veillonellaceae, and Fusobacteriaceae, alongside reductions in Erysipelotrichales, Bacteroidales, and Clostridiales.(4) Interestingly, antibiotic use was found to amplify these imbalances, highlighting how early microbial disturbances may be shaped by both disease and medication. These patterns are echoed across multiple studies. As shown in a recent global systematic review, where increases in potentially harmful bacteria such as Enterobacteriaceae and Bacteroides, and reductions in Faecalibacterium and Oscillospiraceae (known for their anti-inflammatory roles), were consistently reported in people with active Crohn’s disease compared to healthy controls.(5) As well as gut bacteria, research has also shown that the gut fungal ecosystem, or mycobiome, can also be altered in Crohn’s disease, with specific changes linked to inflammation and disease severity. Fungal richness and diversity were found to be significantly higher in inflamed mucosal areas of patients with Crohn’s compared to non-inflamed regions. This shift was mainly marked by expansions in Candida species, Gibberella moniliformis, Alternaria brassicicola, and Cryptococcus neoformans (6), which may all contribute to inflammation and worsen symptoms.
Why Targeting the Microbiome Could Be a Game-Changer for Crohn’s Disease
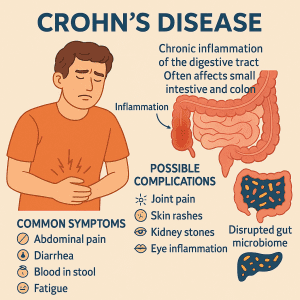 While there is currently no cure for Crohn’s disease, treatment focuses on reducing inflammation, managing symptoms, inducing and maintaining remission, and improving quality of life. This can involve a combination of approaches, including anti-inflammatory drugs, immunomodulators or immunosuppressants, biologic therapies, and targeted synthetic small molecules. In some instances, surgery may be needed to remove damaged areas of the digestive tract.
While there is currently no cure for Crohn’s disease, treatment focuses on reducing inflammation, managing symptoms, inducing and maintaining remission, and improving quality of life. This can involve a combination of approaches, including anti-inflammatory drugs, immunomodulators or immunosuppressants, biologic therapies, and targeted synthetic small molecules. In some instances, surgery may be needed to remove damaged areas of the digestive tract.
However, Crohn’s disease is complex, and while medications can help to control inflammation and relieve symptoms, they don’t always fix underlying issues with the gut microbiome. Research shows that even when patients are in remission, their gut microbiome often remains out of balance (7) which may increase the risk of relapse. That is why precisely targeting the microbiome through personalized approaches could help to maintain longer remission and reduce the risk of flare-ups. This is where microbiome testing comes in. By analyzing the unique microbial community in the gut, microbiome analysis can pinpoint specific imbalances, including increases in harmful bacteria or missing beneficial bacteria. This information helps to tailor interventions, such as targeted diets, probiotics, or lifestyle changes, that actually reshape the microbiome toward a healthier state. Interestingly, emerging research suggests that deliberately changing the gut microbiome might actually help to control underlying inflammation and ease Crohn’s symptoms. For instance, the Crohn’s Disease Exclusion Diet (CDED), which removes dietary components that are thought to negatively impact the gut lining and microbiota, has been shown to induce clinical remission in 62.5% of patients with active Crohn’s within 6 to 12 weeks, supporting its potential as a promising therapeutic approach. 8 Furthermore, the more closely patients stuck to the CDED, the better their outcomes, suggesting that dietary modulation of the microbiome could be a powerful therapeutic tool. Therefore, understanding an individual’s unique gut microbiome through testing provides a powerful tool to guide personalized treatments that could improve Crohn’s outcomes and quality of life.
How Crohn’s and the Gut Microbiome Interact — and Why It Matters

At Enbiosis, we believe that understanding the gut microbiome is key to unlocking better health, especially when it comes to dealing with health conditions such as Crohn’s disease. Our advanced AI-powered gut microbiome testing offers a detailed snapshot of your digestive ecosystem, allowing us to tailor strategies that support your gut health and help to manage Crohn’s disease more effectively.
Visit our website to learn more about our microbiome testing services, or get in touch with us today to find out how we can support you.