Understanding Rheumatoid Arthritis
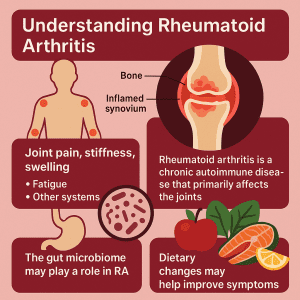
Rheumatoid arthritis (RA) is a chronic autoimmune condition in which the body’s immune system erroneously attacks its own healthy tissues, particularly the joints. The primary target of this misdirected immune attack is the synovium, which is the delicate lining surrounding the joints. As a result, inflammation occurs, leading to pain and, if left unmanaged, progressive joint damage.
RA patients typically experience joint-related symptoms initially, including joint pain, stiffness (particularly in the mornings), swelling, warmth and tenderness of the joints. Over time, as RA is a systemic autoimmune condition, it can progress to affect other areas of the body, including the skin, eyes, lungs, and heart, as well as frequently causing persistent fatigue, intermittent low-grade fever and a general feeling of being unwell.
While we still don’t have all the answers about RA, it is understood to develop in individuals with a genetic predisposition and that exposure to certain environmental factors can initiate or exacerbate the characteristic autoimmune processes. Importantly, recent scientific evidence supports the idea that our gut microbes may affect the development and progression of RA.
Is the Gut Microbiome a Hidden Player in RA?
RA has long been framed as a disease of immune dysregulation, but what if the signals driving that immune disruption don’t originate in the joints at all? Accumulating evidence suggests that our gut may be an active participant in driving the systemic autoimmune responses responsible for the development of RA.
The Gut Microbiome Looks Strikingly Different in RA
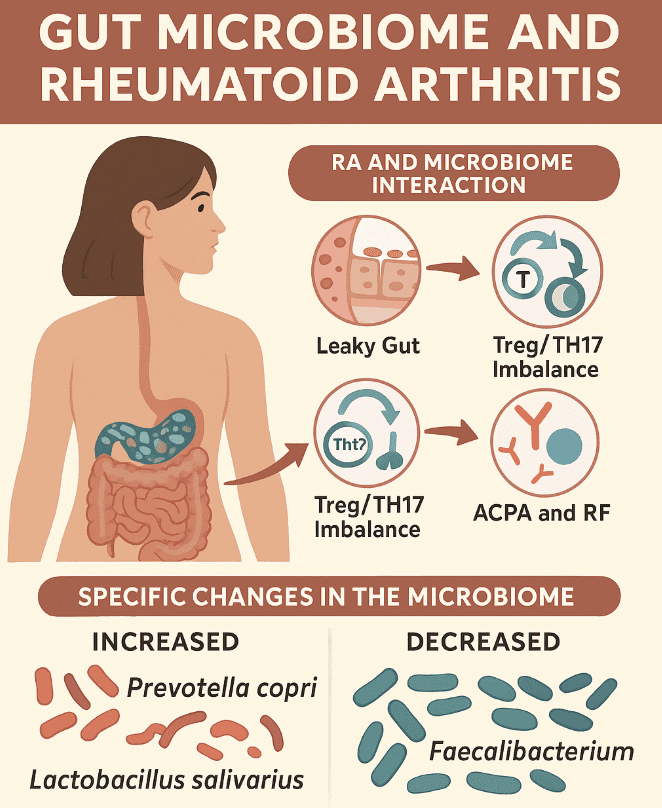
Over the past decade, researchers have uncovered significant alterations in the gut microbiota of individuals with RA and these may occur even before clinical symptoms appear. These shifts typically involve a rise in pro-inflammatory microbes and a drop in those that support immune balance. Notably, at early stages of the condition, an overabundance of Prevotella copri and Lactobacillus has been observed, while levels of beneficial microbes, such as Bacteroidetes, Bifidobacteria and Eubacterium rectale are decreased. (1)
As RA progresses to its active phase, the gut microbial landscape shifts further. Increased levels of Lactobacillus salivarius, Collinsella and Akkermansia have been reported, alongside a marked reduction in Haemophilus species. Additional studies have identified further alterations, including decreases in Faecalibacterium, Fusicatenibacter, Enterococcus and Megamonas, with notable increases in Eggerthellales, Klebsiella, Escherichia, Eisenbergiella and Flavobacterium. (2) These microbial changes appear to correlate with heightened inflammatory activity, suggesting a dynamic relationship between disease state and gut bacteria.
Dysbiosis Fuels Inflammation
An imbalanced gut microbiome doesn’t just influence local gut health — it sets off a chain reaction that can affect the entire immune system. Disruption of the microbial community can compromise the intestinal barrier, allowing bacterial components and antigens to cross into surrounding tissues. This “leaky gut” phenomenon brings microbial signals into contact with the immune system, triggering activation of autoreactive B and T cells in gut-associated lymphoid tissue.
These immune responses disturb the delicate equilibrium between regulatory T cells (Tregs) and pro-inflammatory Th17 cells. The result is systemic immune activation and the production of autoantibodies, including rheumatoid factor (RF) and anti-citrullinated protein antibodies (ACPA), which are central to RA pathogenesis. (3) These autoantibodies may be detected in the blood long before joint symptoms manifest.
Many of these gut changes also affect the production of important microbial metabolites such as short-chain fatty acids (SCFAs), which normally help to regulate inflammation, strengthen the gut barrier and maintain immune balance. A drop in these beneficial metabolites may further tilt the immune system toward autoimmunity. Certain microbes may also express antigens that resemble human proteins, a phenomenon known as molecular mimicry, potentially confusing the immune system further.
From Our Gut to Our Joints
Once the aberrant immune response begins, autoreactive immune cells circulate throughout the body and eventually home in on the synovial tissue of the joints. Specific signals and adhesion molecules on the blood vessel walls near the synovium help these cells exit the bloodstream and enter the joint tissue. There, they initiate a cascade of immune activation that recruits additional immune cells, including macrophages, which become abundant and highly active in the inflamed synovium. (4)
Activated macrophages produce a storm of pro-inflammatory cytokines, including TNF-α, IL-1 and IL-6, as well as tissue-degrading enzymes, such as matrix metalloproteinases (MMPs). The result is progressive cartilage destruction and bone erosion, which are both hallmark features of RA. (5)
Can Dietary Changes Make a Difference?
Understanding the role that our gut microbiome plays in RA is just the beginning. As we uncover more about how our gut bacteria influence systemic inflammation and immune dysfunction, the potential for microbiome testing and personalized dietary interventions becomes clearer. These emerging tools offer hope for more targeted treatments, potentially halting or even reversing the disease process before irreversible joint damage occurs.
Current evidence supports the idea that modulating the gut microbiome could help to improve RA outcomes. For instance, probiotics, particularly certain strains of Lactobacillus and Bifidobacteria, have shown promise as supportive therapies. Among these, L. casei stands out as the most promising strain for reducing RA symptoms. Diets rich in fiber, polyphenols and omega-3 fatty acids, such as the Mediterranean diet, can also support gut health, balance the gut microbiome, optimize the gut barrier and help to reduce systemic inflammation. (6,7)
This evidence suggests that combining personalized nutrition with probiotics and prebiotics may help patients to reduce their RA symptoms, offering a more tailored approach to their treatment.

Can Gut Testing Offer Further Insights for RA?
At Enbiosis, we provide a cutting-edge AI-powered gut microbiome testing service that can help to identify microbial imbalances that are linked to the development and progression of RA. We then use this personalized data to design tailored strategies that target these imbalances. By understanding your unique gut microbiome profile, we can recommend specific dietary modifications, probiotic and prebiotic interventions and lifestyle changes aimed at restoring microbial balance. This targeted approach can help to manage inflammation, reduce symptoms and potentially slow the progression of RA, offering a more precise and effective treatment strategy for each individual.
Visit our website or contact us today to find out more about how gut microbiome testing can transform the management of rheumatoid arthritis.
Contact UsReferences:
1 Zhao, T., Wei, Y., Zhu, Y., Xie, Z., Hai, Q., Li, Z., & Qin, D. (2022). Gut microbiota and rheumatoid arthritis: From pathogenesis to novel therapeutic opportunities. Frontiers in Immunology, 13, 1007165.
2 Coradduzza, D., Bo, M., Congiargiu, A., Azara, E., De Miglio, M. R., Erre, G. L., & Carru, C. (2023). Decoding the Microbiome’s Influence on Rheumatoid Arthritis. Microorganisms, 11(9), 2170.
3 Horta-Baas, G., Romero-Figueroa, S., Montiel-Jarquín, A. J., Pizano-Zárate, M. L., García-Mena, J., & Ramírez-Durán, N. (2017). Intestinal Dysbiosis and Rheumatoid Arthritis: A Link between Gut Microbiota and the Pathogenesis of Rheumatoid Arthritis. Journal of Immunology Research, 2017, 4835189.
4 Szekanecz, Z., & Koch, A. E. (2000). Cell-cell interactions in synovitis: Endothelial cells and immune cell migration. Arthritis Research, 2(5), 368.
5 Kinne, R. W., Bräuer, R., Stuhlmüller, B., Palombo-Kinne, E., & Burmester, R. (2000). Macrophages in rheumatoid arthritis. Arthritis Research, 2(3), 189.
6 Ferro, M., Charneca, S., Dourado, E., Guerreiro, C. S., & Fonseca, J. E. (2021). Probiotic Supplementation for Rheumatoid Arthritis: A Promising Adjuvant Therapy in the Gut Microbiome Era. Frontiers in Pharmacology, 12, 711788.
7 Dourado, E., Ferro, M., Guerreiro, C. S., & Fonseca, J. E. (2020). Diet as a Modulator of Intestinal Microbiota in Rheumatoid Arthritis. Nutrients, 12(11), 3504.